A cleanroom is a room in which the concentration of airborne particles is controlled and constructed and used in a way that minimizes the entry, production, and retention of particles in the room. If necessary, other related factors such as temperature, humidity, and pressure are also controlled in it. This definition of a cleanroom is the most comprehensive definition of a cleanroom defined by ISO 14644.
In accordance with this definition, a cleanroom must b designed, constructed, and used in such a way that it prevents the entry of particles into it, and the materials and materials used in the construction of the cleanroom do not release particles, and finally the particles that are released into the cleanroom by the production process are removed by the cleanroom ventilation system
Application of Cleanrooms in Industries
From the perspective of contamination control technology and cleanrooms, the application of cleanrooms can be considered with three distinct protective objectives:
- Protection of processes and products from the harmful effects of airborne contaminants.
- Protection of workers from exposure to airborne contaminants that can be harmful to health.
- Protection of the outside environment from the release of harmful airborne contaminants from facilities.
Therefore, it can be said that cleanrooms are used in all sensitive and ultra-sensitive industries to pollution, such as pharmaceuticals, medical equipment, electronic equipment, aerospace industries, semiconductor manufacturing, military industries, hospitals, automotive industries, and so on.
read more| History of cleanrooms
Applications of Cleanrooms in Pharmaceutical Industry
Products pharmaceutical must be produced in a way that meets exact standards of efficacy and quality. All aspects of quality are considered in relation to the risks of the route of administration: injectable, oral, etc.; and the way they are produced is also reviewed: the way they are disinfected, sterilized, or under less controlled conditions.
read more| cleanroom equipment
The use of cleanrooms in the pharmaceutical industry is an inevitable necessity to meet the quality and efficacy of pharmaceutical products. The use of cleanrooms in the pharmaceutical industry is not limited t the production area and also includes other areas such as weighing, storage, quarantine, laboratories, etc.
Airflow patterns in cleanrooms
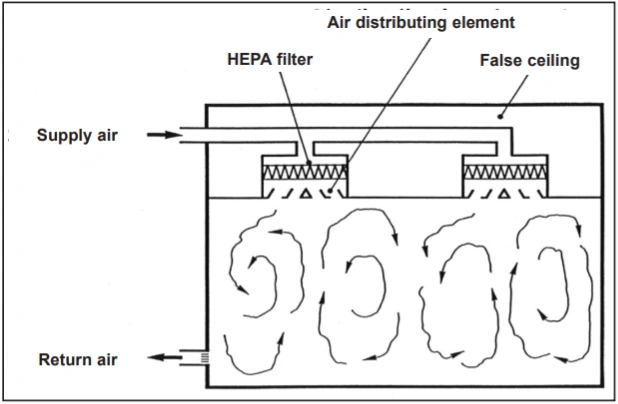
Turbulent Airflow Patterns (Non-Uniform)
A/P/U filters guarantee the desired quality of incoming air to cleanrooms. However, they are not sufficient on their own: To optimally and economically control the effects of particle and microorganism emissions in cleanrooms, an appropriate air flow pattern must be selected. If the air cleanliness requirements of the room are relatively moderate, it is sufficient to dilute the concentration of pollutants emitted in the room with the supply of sufficient amounts of A/P or HEPA filtered air, according to the principle of turbulent air mixing. This air flow pattern (Figure 1) is well known in air conditioning technology. By supplying filtered air with A/P or HEPA filters, it is possible to meet the air cleanliness requirements of ISO Class 7 and 8 rooms in operational mode according to ISO14644-1 using relatively moderate air flow rates.
read more | Applications of cleanrooms in different industries
Proposed Air Filter Combinations for Cleanrooms with Different Cleanliness Grades
| The degree of cleanliness of the air in the room | The first stage of the filter | The second filter stage | The third stage of the filter |
| ISO grade 5 | F7 | F9 | H14 |
| ISO grade 6 | F7 | F9 | H14 |
| ISO grade 7 | F7 | F9 | H13 |
| ISO grade 58 | F7 | F9 | E12 |
Room cleanliness classification according to ISO14644-1 for operational status Filter classification according to EN779:2002 (fine dust filters) and EN1822:2009 (HEPA and ULPA filters) | |||
Unidirectional airflow pattern
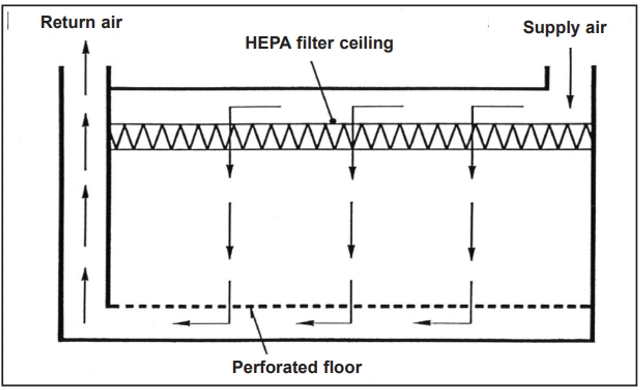
For higher and highest air cleanliness requirements, a different airflow pattern must be used: unidirectional airflow, also known as laminar airflow, although this is not a scientifically accurate term. Unidirectional airflow is typically created by using a continuous HEPA or ULPA filter in the ceiling, which results in airflow moving along more or less parallel flow lines and with a relatively uniform velocity. Therefore, this airflow pattern removes particles and microorganisms released into the room air from the straightest possible path, thus removing them without harm. A characteristic of unidirectional airflow is very low lateral mixing and confines the harmful effects of airborne particles to the same area adjacent to the flow line along which the particle is carried.
read more | Cleanroom standards
Figure 2 shows an idealized example where the airflow is directed vertically downwards and exits through openings in the room floor. For hygienic reasons, such floor coverings cannot be used in the pharmaceutical industry. Therefore, the resulting air must be exhausted from the room through openings in the walls that are located close to the room floor: a somewhat modified airflow pattern is obtained that nevertheless is still capable of sweeping particles in a relatively straight path from their point of release. In unidirectional airflow, the final quality of the air filters must be at least H13, meaning qualitative testing of the penetration rate after installation in place.
1. Airflow patterns in cleanrooms: Turbulent mixed flow
2. Airflow patterns in cleanrooms: Vertical unidirectional airflow
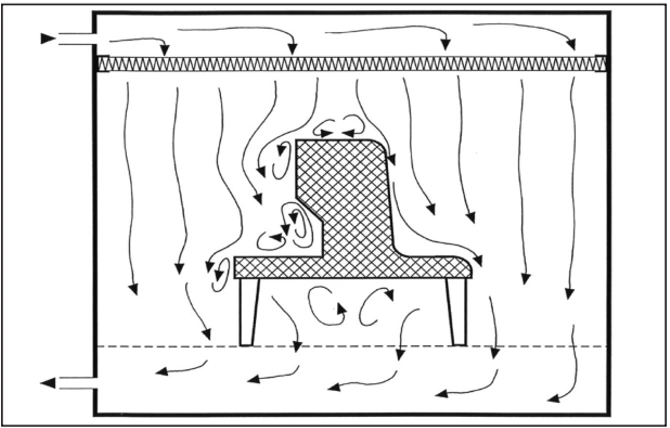
3. Unidirectional airflow around an obstacle – bad (left) good (right)
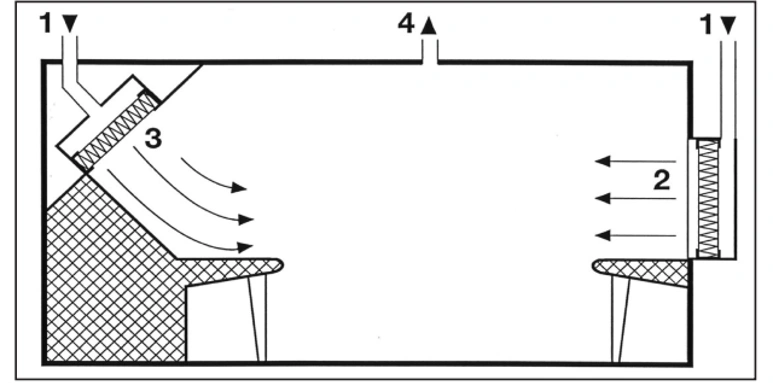
Unfortunately, production equipment installed in cleanrooms that are located in unidirectional airflow zones often do not adequately meet the aerodynamic requirements of this airflow pattern. Therefore, vortices and stagnant air zones may form, both of which allow for lateral transfer of contaminants that are released in those areas. Edges should be rounded and inaccessible angles should be avoided (third point). Local heat sources that produce thermal currents can also cause stagnant air zones (fourth point). Horizontal or inclined airflow (fifth point) can both effectively deal with this phenomenon.
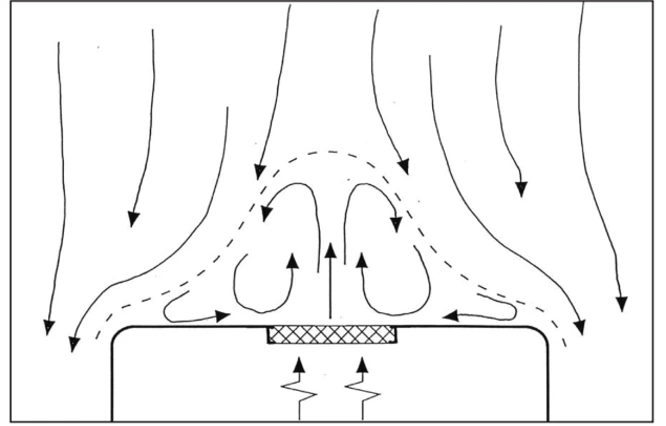
4. Unidirectional airflow deflected by a heat source
5. Control of Heat Sources Using Horizontal or Oblique Unidirectional Air Flow
The air velocity in a stable one-way airflow must be high enough to minimize the effect of local heat sources. Even small temperature differences can cause significant thermal currents: for example, the heat released by a standing or sitting person, with an intensity of about 80 watts, can lead to a thermal current with a velocity of 0.2 meters per second above their head.
read more | Cleanroom Quality Documentation
Cleanroom monitoring
As mentioned, controlled environments (cleanrooms) are required for the production of many sensitive products, especially pharmaceuticals. These rooms must meet a certain standard for the number of airborne particles in order to be assigned a certain cleanliness class. However, once a cleanliness class is assigned, they must also meet a set of other physical and microbiological parameters, including HEPA filtration, pressure differential, wearing appropriate clothing, and so on.
To achieve microbiological control, certain cleaning methods and cleaning and disinfecting agents must be used. To assess the effectiveness of environmental control systems, it is necessary to use a specific environmental monitoring program in the cleanroom, which is an important part of any regulated pharmaceutical quality management system.
read more | Cleanroom Particle Counting Test
Many aspects of drug manufacturing are regulated by Good Manufacturing Practices (GMP). In many cases, these practices are implemented according to specific regulations or guidelines. However, expectations for environmental monitoring are not as well defined. The US Food and Drug Administration (FDA) Guide to Sterile Manufacturing and the European Union (EU) GMP Guideline provide frameworks for sterile manufacturing, although each facility must define its own procedures that are compatible with its conditions. GMPs also specify environmental monitoring expectations in non-sterile manufacturing.
Cleanroom Monitoring Procedures
Cleanroom monitoring procedures can be based on the following guidelines:
- European Union GMP, Annex 1: In the context of sterile production, it provides maximum allowable numbers of viable and non-viable particles, as well as details of accepted methods for environmental monitoring.
- European Union GMP, Section 4-1 “Quality Control”: In the context of monitoring environmental conditions to meet GMP requirements.
- European Union GMP, Section 5: In the context of production facilities that must be protected against “microbial and other contaminations” (5-10), cross-contamination prevention (5-19), and the need for monitoring of process areas (5-20).
- European Union GMP, Annex 7: In the context of preventing contamination during the production of herbal medicinal products
- European Union GMP, Annex 9: Development of warnings regarding the sensitivity of liquids, creams, and ointments to microbial contamination
- Code of Federal Regulations 211.113, “Microbial contamination control,” with an emphasis on the risk of undesirable microorganisms
- FDA guidance on sterile drug products manufactured under sterile processing, September 2004
- USP 35-NF30: Microbial control and monitoring of aseptic processing environments
- ISO 14644 (Parts 1 to 9): Cleanrooms and associated controlled environments
- ISO 14698-1: Cleanrooms and associated controlled environments; Biocontamination control, Part 1: General principles and methods. Geneva, Switzerland, International Organization for Standardization, 2003
This diversity of approach has led to the adoption of a variety of different mechanisms. The purpose of this article is to describe the best practices for cleanroom environmental monitoring and to assist quality professionals in developing and implementing a cleanroom environmental monitoring program, especially for pharmaceutical cleanrooms.
read more | Cleanroom Design
Cleanroom validation
In simple terms, the validation of a cleanroom is the set of operations and documentation that are carried out to verify the quality of the cleanroom. Several test phases are required for the validation of a cleanroom. Special devices and equipment and trained and specialized personnel are required to perform these tests. Ekeas Cleanroom is ready to provide all phases of cleanroom validation with the latest equipment.
read more | Cleanroom Validation
Risk assessment in cleanroom
In recent years, environmental monitoring has shifted from a focus on collecting data for unevaluated purposes to a risk-based approach. Risk-based approaches involve identifying types of risks, assessing their impact by calculating risk severity and likelihood of occurrence, and then accepting or eliminating them. In cases where a risk cannot be eliminated or reduced to an acceptable level, it must be monitored. Environmental monitoring is based on these diagnostic systems. Therefore, pharmaceutical manufacturers are expected to adopt a risk-based approach to microbial and environmental control.
Developing an environmental monitoring program for cleanrooms is one of the most important considerations for microbiology and quality assurance professionals. This requires a sound logical basis (including policies and procedures), with an emphasis on monitoring, data analysis, trend identification, and follow-up actions in relation to out-of-specification results.
read more | What is a HEPA filter
This article considers environmental monitoring of cleanrooms in its broadest sense. This scope can be divided into three areas:
- Physical performance and certification of cleanrooms
- Control of the number of non-viable particles in cleanrooms
- Control of live microorganisms in cleanrooms
As mentioned above, there is a significant distinction between environmental monitoring and environmental control. The physical performance of cleanrooms, along with management oversight and trained and qualified personnel, provide the control aspect. The key point in this regard relates to the design and associated risks that are associated with specific sections of process operations. Methods related to viable and non-viable particles provide the monitoring aspect. Such data help to understand the current performance of the facility.
In environmental monitoring of cleanrooms, it is important to note that monitoring provides only a “snapshot” of the cleanroom process conditions at a specific point in time. Each of the events is rarely important in itself. What is important is the trend path and the assurance criteria and the speed of addressing the identified contamination.
Sources of contamination in cleanrooms include two cases: responsibilities and cleanroom personnel.
responsibility
A set of responsibilities exist with regard to environmental monitoring and control of cleanrooms. According to current GMPs, these roles should be defined. The role of quality assurance requires the development of standards, policies, and monitoring of operations. This includes changing monitoring controls at times of cleanroom renovations or for new buildings; it also requires monitoring of instances where functional aspects of processing can impact the room environment (such as the use of a grinder that produces higher levels of particles); in major contamination events, it also requires monitoring of changes made to control contamination.
Engineers are responsible for helping to ensure that the design and operation of cleanrooms meet required standards. Production staff are responsible for using cleanrooms properly and evaluating physical performance aspects. The role of microbiology specialists is to develop and lead an environmental monitoring program for viable and non-viable particles in a cleanroom. This includes identifying monitoring locations, setting alarm and action levels, identifying trends and interpreting data, providing training, reviewing new equipment required for the process, and reviewing out-of-range results.
read more | Cleanroom softwall
Cleanroom Standards and Classification
Products sensitive to contamination must be manufactured to meet stringent efficacy and quality standards. All quality aspects are assessed in accordance with the risks of the route of administration: injectable, oral, etc.; and how they are manufactured: disinfected, terminally sterilized, or under less controlled conditions.
Cleanrooms are classified according to the number of suspended particles per unit volume of air. There are different standards for cleanrooms, but the most common one is ISO 14644-1. This standard classifies cleanrooms into seven classes, from class 1 with the lowest level of contamination to class 7 with the highest level of contamination.
read more | Air-cooled chillers
Cleanroom Workers
Employees are the primary source of microbial contamination in cleanrooms, including both production workers and other personnel present in the process areas, such as janitors and engineers. Therefore, it is important that all personnel entering cleanrooms are well-trained in proper behavior and gowning procedures. Other training should also be provided on hygiene and basic microbiology.
Cleanrooms and the Identification of Sources of Contamination
Cleanrooms and zones are environments designed to control the concentration of airborne particles. This article and other articles on cleanrooms at Akyas provide details about clea rooms. Cleanrooms are designed to minimize the ingress, generation, and retention of particles. The cleanliness level of a cleanroom is determined by the number of particles present in the air of the cleanroom. The HVAC (heating, ventilation, and air conditioning) system controls the cleanliness level of the room. The key point is to control the cleanliness level of the room.
In addition, cleanrooms are designed to control the pressure of the cleanroom, the volume of air entering the cleanroom, the temperature and humidity of the cleanroom. Therefore, the design, construction, and operation of cleanrooms are important in environmental control.
Cleanrooms are designed to minimize contamination and control its level. There are many sources of contamination. Ambient air contains dust particles, microorganisms, condensates, and gases. Production processes also produce a wide range of pollutants. Processes that involve grinding, abrasion, steaming, heat generation, spraying, rotation, etc., produce particles and dust and cause contamination of the surrounding environment. Contamination can lead to costly downtime and increased production costs.
read more | Hygienic air conditioner
The cleanroom must also be maintained and cleaned to the same high standards after construction. It has been found that the majority of these pollutants are produced from five main sources: facilities, people, tools, fluids, and products in production can all play a role in creating contamination.
Employees of clean environments contribute the most to contamination and cause the release of body vapors, dead skin, microorganisms, skin oils, etc. On average, 1,000,000,000 skin cells are shed from the human body every day, 10% of which contain microorganisms. Humans naturally produce a lot of particles. Most of these particles are created by the outer layer of our skin surface, but they may also enter the surrounding air through our mouths and noses when we breathe, cough, sneeze, laugh, sing, and shout. Particles that come out of people’s mouths and noses are small liquid droplets that are heavily contaminated with microorganisms. This shows the importance of wearing clean clothes and how to wear them properly.
Pollutants are of significant importance in the field of contamination control and cleanroom technology. The figure below shows a schematic overview of the source, release, and deposition of pollutants. This figure shows four general sources of different pollutants. In addition,
Relationship between the four main sources of contamination: personnel, surfaces, air, and production process. The lines connecting the sources of contamination show the paths of contamination deposition. In fact, the sources of contamination are the same places where contamination can be deposited.
The four main sources of contamination in a cleanroom are: personnel (i.e., humans), surrounding air, all surfaces inside the room, products, or the process itself. These sources are all interrelated. From a general perspective, all four sources are equally important. However, from a practical perspective, the question often arises, “Which of these four sources are the most important in relation to the work being done?” The answer to this question is that all four are important, but the impact of each one on the outcome of the production process depends on the nature of the product, its sensitivity, and also how the product is used.
read more | Standard air conditioner
Personnel can contaminate the product or production process through air, direct or indirect contact with various surfaces, and also direct contact with the product or process equipment. As mentioned, the most important contaminating effect that people can have is through the natural process of regular shedding of epidermal cells. It is said that each person in a sitting and still state releases approximately 100,000 particles per minute. When moving, even if it is small, the number of particles increases significantly. Most cleanroom microorganisms are in the air. If they sit on a dry surface, they are unlikely to survive and, ideally, any contamination is removed from the room.
There are various clean air devices in cleanrooms. In the vocabulary section of ISO 14644-7 (Cleanrooms and Associated Controlled Environments, Part 7), the term “barrier devices” is used to describe clean air hoods, glove boxes or gloveboxes, isolators, and small environments. These devices include laminar airflow (which in the field of drug production is called unidirectional airflow devices because it is not easy to create “true” laminar airflow), biosafety cabinets, and isolators. Such devices are typically EU GMP Grade A or ISO Class 5 compliant. The term “cabinet” is more common in Europe and the term “hood” is more common in the United States.
Cleaning and disinfection of a cleanroom
Cleanrooms and areas must be regularly cleaned and disinfected. This is typically done in two steps, first cleaning with a detergent and then disinfecting with an appropriate agent. It may be necessary to remove any residual disinfectant with water.
read more | Cleanroom stainless steel equipment
Cleaning equipment (mops and buckets) must be designed for the cleanliness class of the room. Strict cleaning procedures must be followed. When cleaning and disinfecting a cleanroom with a mop and cloth, it is best to wet the cloth and clean the area using overlapping parallel strokes (with approximately one-quarter overlap); never use circular strokes. The direction of cleaning should be toward the user (top to bottom, back to front). To prevent excessive concentration of cleaning or disinfecting agents, they should only be used once. Cleaning and disinfection should start in the “cleanest” area and proceed to the “dirtiest” area.
Usually, cleaning is performed in each operating area before use. In general, risk assessment determines the time interval between subsequent cleanings. The environmental monitoring program provides useful information for evaluating the effectiveness of cleaning and disinfection programs (especially surface monitoring).
Cleanroom qualification
Cleanroom qualification documentation includes the following five items, which are fully discussed in this article on the Ekias cleanroom website:
- Design Qualification (DQ)
- Installation Qualification (IQ)
- Operation Qualification (OQ)
- Performance Qualification (PQ)
Cleanroom Environmental Monitoring Program: Nonviable Particle Count
Particle monitoring is an important part of a cleanroom environmental monitoring program. This monitoring goes beyond the classification of the cleanroom.
A “particle” in the context of cleanrooms is a general term for small visible materials. It can be liquid or solid and can range in size from 0.001 to 1000 micrometers. The term “fiber” may also need to be defined. In general, a fiber is defined as a particle with a length-to-diameter ratio of 10 or greater. Airborne particles are particles that are suspended in the air. There are many different particles of varying sizes in the air, which can be dust particles, skin, microorganisms, and so on. As explained, the function of cleanrooms is to reduce the number of airborne particles. For example, there are 500,000 to 1,000,000 particles larger than or equal to 0.5 micrometers in every cubic foot of air in an office building. However, a Class A GMP EU or ISO Class 5 cleanroom is designed to have no more than 100 particles (0.5 micrometers or larger) per cubic foot of air. The diameter of a human hair is about 75-100 micrometers. A particle with a diameter 200 times smaller (0.5 micrometers) than a human hair can be disastrous in a cleanroom.
Particles can have various sources, including:
- Cleanroom facilities; such as walls, ceilings, paint and coatings, building materials, particles that pass through the HVAC system, room air and vapors, spills and leaks;
- People; including skin flakes and oils; cosmetics and perfume; saliva; fibers and lint that come off of clothing; hair, and so on;
- Cleanroom equipment, including: particles that are generated by friction and wear, lubricants and gases produced, vibrations;
- Cleanroom cleaning equipment such as: brooms, mops, dustpans, and cleaning chemicals;
- Fluids; which are due to leaks;
- Airborne particles, primarily: bacteria, fungi, organic matter, and moisture;
- Particles generated by the product.
The unit of measurement for particles is “micrometer” or “micron”. Its symbol is µm. A micron is a length unit equal to one millionth (10^-6) of a meter. Cleanroom regulatory standards focus on two particle sizes that have been selected because of the potential risk they pose. These two sizes are:
- Particles with a size of 0.5 micrometers, which are similar in size to many microorganisms;
- Particles with a size of 0.1 micrometers, which are similar in size to skin flakes, which many microorganisms attach to.
EU GMP considers both sizes, but the primary focus of the FDA guidance is on 0.5-micrometer particles.
Particle evaluation during cleanroom classification is an indicator that the physical aspects of the cleanroom operation are working correctly, whatever they may be. In day-to-day operations, the purpose of particle counting is to assess the impact of equipment and personnel on the product and process of interest.
The frequency of particle counting depends on the nature of the operations. According to EU GMP and FDA guidance, continuous particle counting is required for the packaging of products under aseptic conditions. For other manufacturing activities, risk assessment determines how the counting sequence will be.
To this end, the following can be done:
- List areas and operations that are not monitored (such as operations that are known to produce high levels of particles, such as centrifugation. It may be necessary to show that particles produced by certain materials or specific equipment are non-microbial. To do this, the particle level inside the cleanroom can be measured when the equipment is not running and people are not present, and then measured again after the equipment starts running and still without people present, and finally the differences can be compared.);
- Focus on downstream operations such as purification, final formulation, and primary packaging (preparation of components);
- Determine the frequency of monitoring (one approach is to perform measurements at short intervals and, if the data are satisfactory, increase the intervals to one month).
Monitoring locations can be determined on the following basis:
- By reviewing the results of the particle count study for classification and identifying the location that was the “worst case” (if any);
- By reviewing the process flow and selecting tests that are representative of the operations;
- By using risk assessment. For example, does the operation in question have a negative impact on the adjacent cleanroom (perhaps with a different class)? In such an example, a particle counter may need to be placed in the adjacent area.
Cleanroom Design
Cleanroom design is the process of creating a set of documents, including notes and drawings, that provide the user with an understanding of the future production facility and allow them to estimate the costs and how to build it. Cleanroom design consists of two phases, preliminary design and working drawings.
Cleanroom Filters
Cleanrooms Built by Ekeas Cleanroom
Cleanroom Equipment by Ekeas
Cleanrooms include a variety of equipment, and in order for a cleanroom to meet standards, it must use the appropriate equipment. Here are a few examples of cleanroom equipment: